Formaldehyde (HCHO) is a dangerous volatile organic compound (VOC) that is present in indoor environment such as houses, offices and schools. HCHO emanate from wood adhesives, furniture, antiseptics and disinfectants, textiles, dyes, cigarette smoke, etc. Its slow release in indoor air can be toxic and carcinogenic to humans, even from exposure to very low concentrations ranging from 3 ppb to 0.1ppm. Therefore, the abatement of this pollutant is of significant practical interest to improve our indoor air quality. HCHO can be removed from air by various methods such as adsorption, membrane separation, condensation, combustion or photocatalytic oxidation, however heterogeneous catalytic oxidation is the most promising way. This process completely oxidizes the organic pollutant into non-harmful molecules such as CO2 and H2O, with low energy consumption.
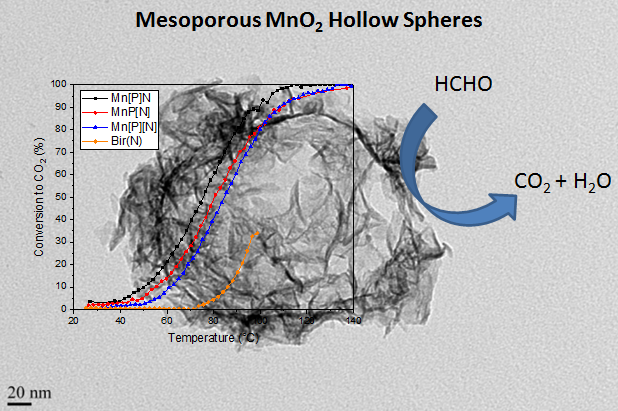
This work reports on a new low temperature method to synthesize hollow MnO2 spheres using a templating approach. This method is much more advantageous than the classical hydrothermal method, due to the high temperature and reaction time required in the latter. The synthesized hollow spheres had very high surface areas and different crystal phases could be obtained from simple changes in the synthesis method. The catalysts showed the high activity in catalytic oxidation of formaldehyde in dry air. Long-term stability tests showed that γ-MnO2 deactivated gradually with time potentially due to structural collapse of the hollow spheres. The interlayer spacing in the δ-MnO2 sample, however, was responsible for a higher than expected conversion, possibly due to the regeneration of oxygen/hydroxyl species from adsorbed water formed from the oxidation of formaldehyde on the catalyst surface. (Sustainable Materials and Technologies, 2018, 3, in press. Corresponding author)